Unit 11: The Atomic Nature of Matter
All matter around us consists of tiny building blocks called molecules, and each molecule is either a single atom or a cluster of atoms. Each atom consists of smaller particles: protons, neutrons, and electrons. Physicists believe there is another structural level of even smaller particles, referred to as quarks, which are the building blocks of protons and neutrons. After completing this unit, you should be able to explain the atomic structure of matter and discuss its implications.
Learning Outcomes
By the end of this unit, you should be able to
- state the atomic hypothesis.
- describe atomic structure and the characteristics of atoms.
- discuss the diversity and classification of atoms, and the periodic table of the elements.
- define isotopes.
- explain the difference between mixtures and compounds.
- explain the difference between atoms and molecules.
- explain the difference between matter and antimatter.
eText Material
Reading Assignment
Read the following sections in Chapter 11 of the eText:
- 11.1: The Atomic Hypothesis
- 11.2: Characteristics of Atoms
- 11.3: Atomic Imagery
- 11.4: Atomic Structure
- 11.5: The Periodic Table of the Elements
- 11.6: Isotopes
- 11.7: Molecules
- 11.8: Compounds and Mixtures
- 11.9: Antimatter
Supplementary learning resources are available on the Mastering Physics learning platform.
Additional Reading
Atomic Structure
As physicists began to probe the world on the scale of individual atoms, crude models of the atom began to take shape. After discovering the electron, J. J. Thomson proposed the plum-pudding model of the atom. Ernest Rutherford’s alpha-particle-scattering experiments led to his planetary model, which had electrons orbiting a dense positive nucleus. Despite a growing knowledge of what made up an atom, classical physics could not explain how atoms worked.
It is now common knowledge that an atom consists of a positively charged nucleus surrounded by a cloud of negatively charged electrons. Normally, atoms are electrically neutral because the positive and negative charges are balanced. Under certain circumstances, an atom may lose or gain electrons, thus making it electrically charged. A positively or negatively charged atom is referred to as an ion.
Almost all the mass of an atom is concentrated in its nucleus, which is a bound state of two types of subatomic particles: positively charged protons and electrically neutral neutrons. All atoms of the same element contain an equal number of protons but may have different numbers of neutrons. These different atoms of the same element are called isotopes. For example, uranium-238 is the most common isotope of the uranium element, where its nucleus contains 92 protons and 146 neutrons. Another important isotope (but with an abundance of less than 1%) is the lighter uranium-235, which has 92 protons and 143 neutrons. Uranium-235 is used as a fuel in nuclear power plants and is a component of nuclear weapons.
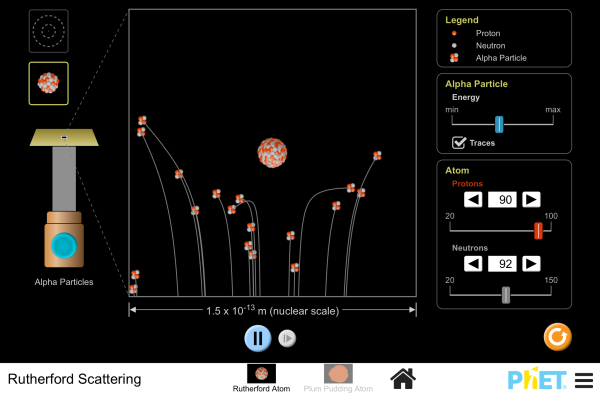
Simulation
Run the Rutherford Scattering simulation, which simulates the experiment used to disproved the plum-pudding model of the atom. Rutherford observed the alpha particles bouncing off atoms and determined that atoms must have a small core.
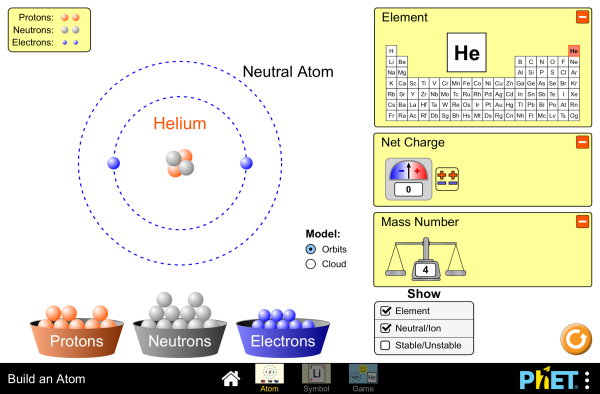
Run the Build an Atom simulation to build an atom out of protons, neutrons, and electrons and see how the element, charge, and mass change.
Simulations by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0 (https://phet.colorado.edu)
Chemical Versus Nuclear Reactions
Chemical reactions involve the breaking of bonds between atoms and the formation of new bonds. Note, however, that such rearrangements of atoms into new molecular structures involve only the electron component of the atoms; the nuclei remain unchanged. Therefore, it is not possible to convert an element into a different element (e.g., carbon into nitrogen) in a chemical reaction. For such a conversion to take place, the number of protons in the nucleus must be altered, which requires a different type of reaction, called a nuclear reaction.
Protons and neutrons are nearly equal in mass and are glued together inside the nucleus by the strong nuclear force. In one type of nuclear reaction called beta decay, a neutron changes into a proton. In the process, an electron and another particle called an antineutrino are emitted. Consequently, the number of protons in the nucleus increases by one and the atom becomes an isotope of the next element in the periodic table.
Antimatter
For every elementary particle discovered, there is an antiparticle that has the same mass and carries an equal but opposite charge. For example, the antiparticle of the electron is called the positron. There are also the negatively charged antiproton and the neutral antineutron. When a particle and its antiparticle interact, both annihilate and convert into photons—energy packets of electromagnetic radiation.
Questions
The following questions are selected from the end of Chapter 11 of the eText. It is important to your learning that you try to answer each question independently before you read through the answer and explanation given.
For questions that ask you to explain or discuss your answer, the response revealed by the Answer button would earn you only partial marks on a quiz or exam in this course. Use the Answer to help you formulate a complete answer before you select the Explanation button to check your work.
Chapter 11
Question 2
What causes dust particles and tiny grains of soot to move with Brownian motion?
Answer
Collisions with moving atoms.
Explanation
Brownian motion is caused by collisions between tiny dust specs or soot grains (visible through a microscope) and fast-moving invisible atoms (or molecules) in a gas or liquid. Brownian motion demonstrates the existence of atoms, as well as their perpetual erratic motion.
Chapter 11
Question 11
What is the meaning of the term nucleon?
Answer
A nucleon is a proton or neutron.
Explanation
The components of the nucleus are called nucleons, which consist of positively charged protons and uncharged neutrons. Note that a proton and a neutron have approximately equal masses.
Chapter 11
Question 38
Rank the mass of these molecules from most to least.
Figure Q11.38
Answer
A, B, D, C.
Explanation
By referring to the periodic table, you can calculate the mass number of each molecule in Figure Q11.38: \begin{align} \text{A. } & \text{Oxygen molecule,}\; \text{O}_2 \text{:} & 2 \times 16 = 32 \nonumber\\ \text{B. } & \text{Water molecule,}\; \text{H}_2\text{O} \text{:} & (2 \times 1) + 16 = 18 \nonumber\\ \text{C. } & \text{Methane molecule,}\; \text{CH}_4 \text{:} & 12 + (4 \times 1) = 16 \nonumber\\ \text{D. } & \text{Ammonia molecule,}\; \text{NH}_3 \text{:} & 14 + (3 \times 1) = 17 \nonumber\\ \end{align}
Chapter 11
Question 42
How many types of atoms are in a pure sample of any element?
Answer
One
Explanation
The term element refers to a substance composed of a single type of atom. Therefore, an element (such as oxygen, mercury, or gold) cannot be broken down into simpler substances. It is possible, however, for the sample to include different isotopes of the same element.
Chapter 11
Question 44
The average speed of a perfume-vapor molecule at room temperature may be about 300 m/s, but you’ll find the speed at which the scent travels across the room is much less. Why?
Answer
Because of the many collisions of the perfume-vapor molecules with the air molecules.
Explanation
Although a perfume-vapor molecule may have a relatively high speed, its path is full of obstacles. It continuously bumps into air molecules that are moving in random directions, causing the perfume-vapor molecule to change its speed and direction of motion after each collision. So, overall, perfume-vapor molecules that are initially more localized diffuse through air more slowly than the speed of individual molecules.
Chapter 11
Question 47
How many elements are in a molecule of sulfuric acid, H2SO4? How many atoms?
Answer
There are three elements and seven atoms.
Explanation
A sulfuric acid molecule (H2SO4) consists of two atoms of the hydrogen element, one atom of the sulfur element, and four atoms of the oxygen element.
Chapter 11
Question 50
The mass numbers of two isotopes of cobalt are 59 and 60.
- How many protons and how many neutrons are in each isotope?
- How many orbiting electrons does an atom of each have when the atoms are electrically neutral?
Answer
- Cobalt-59 has 27 protons and 32 neutrons. Cobalt-60 has 27 protons and 33 neutrons.
- 27.
Explanation
- Referring to the periodic table, you see that the atomic number for the element of cobalt is 27, which is equal to the number of protons inside the nucleus. Since all isotopes of cobalt have 27 protons in their nuclei, the lighter isotope (mass number 59) has $59 - 27 = 32\,\text{neutrons}$ and the heavier isotope (mass number 60) has $60 - 27 = 33\,\text{neutrons.}$
- For an atom to be neutral, the number of electrons must be equal to the number of protons. Therefore, any neutral isotope of cobalt must have 27 electrons.
Chapter 11
Question 55
The noble gas helium is an inert gas, meaning that it doesn’t combine with other elements. Name five other noble gases. (See the periodic table.)
Answer
Ne, Ar, Kr, Xe, and Rn.
Explanation
Elements with similar properties are located in the same column in the periodic table. The five other inert gases are located in the last column under helium: neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Chapter 11
Question 59
A hydrogen atom and a carbon atom move at the same speed. Explain why one of them has a greater kinetic energy than the other.
Hint
In the periodic table of the elements, look up the mass number for each atom.
Answer
The kinetic energy of a particle of mass $m$ and speed $v$ is calculated using the formula \begin{equation} \text{KE} = \frac{1}{2} m v^2 \end{equation} Since the mass of a carbon atom is 12 times greater than the mass of a hydrogen atom, their kinetic energies also differ by the same ratio when the atoms are moving at the same speed. The carbon atom therefore has greater kinetic energy.
Chapter 11
Question 73
Discuss which contains more atoms: 1 kg of lead or 1 kg of aluminum.
Answer
The 1 kg of aluminum contains more atoms. The mass number of aluminum is 27, while for lead it is 208. Since the lead atom is approximately eight times heavier than the aluminum atom, 1 kg of aluminum has about eight times more atoms that 1 kg of lead.
Chapter 11
Question 79
Somebody told your friend that if an antimatter alien ever set foot upon Earth, the whole world would explode into pure radiant energy. Your friend looks to you for verification or refutation of this claim. What do you say?
Answer
This claim is not accurate.
Explanation
A certain amount of antimatter of mass $m$ will annihilate with only an equal amount of matter and convert to radiation. The total radiation energy released as a result is equal to $2 m c^2$.
Suppose the mass of the antimatter alien is 60 kg. The energy released as the alien steps on the ground would be \begin{equation} 2\, (60\,\text{kg})\, (3 \times 10^8\,\text{m/s})^2 = 1.1 \times 10^{19}\,\text{J} \nonumber \end{equation} This is an enormous amount of energy—more than 50 times the energy released when the Soviet Union detonated the 50-megaton Tsar Bomba on October 30, 1961. It would be a huge catastrophe, but the world would not explode into pure radiant energy.
Additional Questions
Answer the following additional questions (not found in your eText).
Unit 11
Question A
Explain how an ion is different from a neutral atom.
Answer
A neutral atom carries an equal number of protons and electrons, which results in zero net charge on the atom. Since the electron carries a negative charge, an extra electron transforms a neutral atom into a negatively charged ion. A positive ion is produced when a neutral atom loses an electron.
Unit 11
Question B
If two protons and one neutron are added to the nucleus of a boron-11 atom, what is the resulting atom?
Answer
Nitrogen-14
Explanation
When two protons are added to the nucleus of an element, its atomic number increases by two. Since the atomic number of boron is 5, the resulting atom after adding two protons is that of nitrogen (atomic number 7).
Also, when two protons and one neutron (i.e., three nucleons) are added to a boron-11 nucleus, its mass number increases by three. Therefore, the mass number of the resulting nitrogen nucleus is $11 + 3 = 14$. \begin{align} \text{Boron-} &11 \nonumber\\[6pt] &\Downarrow\; \text{add two protons} \nonumber\\[6pt] \text{Nitrogen-} &13 \nonumber\\[6pt] & \Downarrow\; \text{add one neutron} \nonumber\\[6pt] \text{Nitrogen-} &14 \nonumber \end{align}
Exercises
Spend some time completing the following exercises to test your understanding of the main concepts in Chapter 11 and increase your efficiency in answering exam questions.
End-of-Chapter Practice Questions
Answer questions 3, 7, 9, 17, 23, 29, 37, 41, 43, 45, 51, 53, 57, 69, and 75 from Chapter 11 of the eText. If you require assistance, please contact your tutor. The answers are provided at the end of the eText.